Charcoal production process
The raw material: wood

The installation of a charcoal production unit can be done near a forest exploitation or a sawmill, because Blu Karb uses industrial and forestry wood waste to produce its charcoal.

Blu Karb also recovers non-market wood, such as branches which represent around 30% of a tree, for the production of its charcoal.
This wood waste is valued as charcoal, which prevents them from releasing CO2 by decomposing. This is another way of fighting greenhouse gas (GHG) emissions.
Steps to achieve charcoal production
The production of charcoal follows the following process:
- wood production: planting, management, sustainable development,
- wood harvesting (exploitation, thinning) and recovery of wood waste,
- storage and drying of wooden trunks and waste,
- transformation into wood chips and silo for drying before carbonization,
- carbonization of wood in a controlled manner to obtain charcoal,
- unloading of kilns, storage and bagging.
How is wood made into charcoal ?
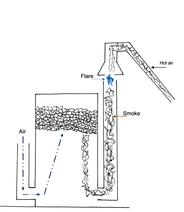
The charcoal production process, implemented by the "MAGE" technology, works according to an inverted draft (see diagram opposite), which makes it possible to burn the gases emitted in a chimney provided for this purpose. The gases are destroyed by a flare located at the top of the chimney, and the resulting hot air is conveyed in a silo to pre-dry the wood which will be transformed into charcoal during the next charring.
- The wood chips are loaded in the kiln,
- The air penetrates uniformly in the wood load for a homogeneous combustion,
- The chips are of a homogeneous size (5*2*2 inches),
- The ignition is carried out with small wood from the top of the oven; the technician then closes the cover and leaves the air inlets open, which produces a homogeneous descent of the fire from top to bottom.
The ignition phase is thus completed, as is the carbonization cycle to obtain charcoal.

The spread of the kindling of small wood to the entire load constitutes the beginning of the combustion phase. Different reactions (described below) occur to complete the production of charcoal by carbonization : first endothermy, then exothermic and finally again endothermy.
During these different phases, the hot air that leaves the chimneys is directed to the wood pre-drying silos, in order to reduce the humidity level of the wood chips and thus accelerate the charcoal production process. Once the last reaction is complete, the kiln remains at rest for 4 to 6 hours, until the temperature drops to around 140°F.
The charcoal obtained is then poured and stored in dampers until complete cooling. The last phase is bagging, before transport for local shipment or export.
Why do we get charcoal ?
What is wood made of ?
Wood is made up of 3 main substances: cellulose, lignin and water. The first two are strongly linked together to constitute the material called "wood". Water is absorbed, that is to say retained in the form of molecules on the surface of the cellulose / lignin complex.
Air-drying wood still contains 20-30% of absorbed water. Newly cut or "green" wood also contains water in liquid form, which gives it a total moisture content of 40 to 100%, expressed as a percentage of the weight of anhydrous wood.
This water will have to be eliminated as much as possible, it is therefore necessary to store the wood to eliminate a part of it, then to make it pre-dry in a silo in order to improve the profitability of charcoal production.
What are the steps to achieve charcoal ?
- Up to 320°F, the wood almost only loses water.
- Up to 390°F, the wood becomes brown, it loses its humidity, as well as acetic acid and other volatile compounds carried away by water vapor.
- From 390°F to 520/535°F, oxygenated gases are released : CO, CO2, water vapor, methanol, acetic acid. This results in red wood, also called roasted wood, but which is not yet charcoal. It is the 1st endothermic reaction, ie absorbing energy.
- From 520/535°F, the wood begins to decompose spontaneously, with a view to an exothermic reaction, which raises the temperature to around 660/715°F, without any external supply of calories. This results in charcoal, with still oxygenated gases but in a smaller quantity, and hydrocarbons whose molecular weight is low (ethane, methane, ethylene).
- The process continues until all of the wood is turned into charcoal and, in the case of pyrolysis, stops at this stage. The charcoal produced then has a content of 65-75% of fixed carbon. Tar residues can represent up to 30%.
- The "MAGE" process being able to control the temperature above 750°F, it produces a charcoal whose fixed carbon content increases, which causes the destruction of more tars. The gases that form are largely made up of hydrocarbons, and the pyroligneous juices are enriched with heavy tars.
- When reaching 930°F, the fixed carbon content in charcoal reaches around 85% and volatile elements 10%, this is the 2nd endothermic reaction.
